From the Bench: Molecular Jackhammers, Biobots, & More

For our readers who enjoy learning about the newest tools and strategies in cancer research, the third installment of our series, “From the Bench,” is here. For more cutting-edge advances in cancer research technology, check out our summer and fall editions from 2023. This quarter, we bring you molecular jackhammers that can blast apart cancer cells, CAR T cells that become active in low-oxygen environments, a wearable ultrasound for enhanced breast imaging, biobots made from human cells, and a way to track cancer cells through both time and space.
Going Off-air: CAR T Cells That Thrive in Hypoxia
Blood cancers express several cancer-specific proteins on their cell surfaces, some of which are targeted by FDA-approved chimeric antigen receptor (CAR) T-cell therapies. Researchers have struggled to design CAR T cells that effectively target solid tumors, in part, because the targets expressed on the surface of solid tumors are also commonly expressed by cells that make up healthy tissue in vital organs. Targeting these proteins can, therefore, cause fatal off-target effects.
In an article published in the AACR journal Cancer Research, researchers explored an alternative strategy to direct CAR T cells to solid tumors—by taking advantage of their hypoxic (oxygen-deplete) environments.
The researchers designed the CAR T cells so that the gene encoding the CAR—which is required to jumpstart antitumor immune activity—would only be expressed under hypoxic conditions. Since most healthy cells reside in environments with typical oxygen levels, this design ensures the CAR is expressed primarily in cancer cells of solid tumors. Further, the researchers maintained the CAR T cells in a resting state during the manufacturing process to limit the risk of T-cell exhaustion in vivo.
Using preclinical tumor models, the researchers demonstrated that hypoxia-responsive CAR T cells had higher proliferation rates, greater antitumor activity, and lesser exhaustion than conventional CAR T cells. Moreover, the hypoxia-responsive CAR T cells had lower CAR expression and cytokine secretion under normal oxygen levels, suggesting they may be less likely to target healthy tissue.
The authors proposed that their design could help improve the safety and efficacy of CAR T-cell therapy for solid tumors.
Microscopic “Biobots” Can Crawl, Spin, and Heal Wounds
Tiny, spherical assemblies of cells called organoids are frequently used to research human tissues, but making human organoids move or perform functions without genetic manipulation has proven difficult. In a recent study in Advanced Science, researchers provided a proof of concept that “biobots,” mobile spheroids that can be coaxed to self-assemble from human seed cells, can be produced with different sizes and functions.
To produce these biobots, the researchers started by embedding human bronchial cells—which have hair-like structures called cilia that help cells move—into a gel-based matrix and letting them grow into spheroids. Then they relocated the spheroids to an aqueous medium, which resulted in cilia localized on the outer surface, enabling the biobots to move independently. The researchers comprehensively characterized the size, morphology, and movement behaviors of hundreds of biobots and discerned a consistent relationship between structure and function. Perfectly spherical biobots with evenly distributed cilia did not move, while biobots with bilateral symmetry tended to move in straight lines, and biobots with irregular shapes tended to move in circular patterns.
When plated alongside a monolayer of human neurons that had been scratched to form a wound, both linear-moving and circular-moving biobots were able to traverse the wound. When the researchers created superstructures composed of several biobots and used them to bridge the wound, the “superbot” bridge promoted wound closure within 72 hours. Given these impressive properties observed by wild-type biobots that have not been genetically manipulated nor subjected to chemical stimuli, the authors speculated that engineered biobots could have a variety of potential applications in medicine, including targeted drug delivery.
Cracking Cancer With Molecular Jackhammers
Jackhammers can tear apart city sidewalks … but can they destroy cancer cells too?
Not quite, but molecules that function similarly to jackhammers might, according to recent research published in Nature Chemistry. In this article, researchers described the development of an aminocyanine molecule that embeds itself into cell membranes. When exposed to a certain wavelength of infrared light, the aminocyanine vibrates violently—almost like a jackhammer—and tears up the cell membrane in the process, killing the cell.
Using mouse models of melanoma, the researchers showed that injection of the aminocyanine into a melanoma lesion and subsequent exposure to infrared light led to dramatic reduction of tumor growth. Mice that underwent this treatment also survived longer than mice that either received a control injection or were not exposed to the infrared light. The authors concluded that mechanical disruption of cancer cells using this strategy has potential for cancer treatment.
Ultrasound in a Bra: Conformable Patch for Breast Imaging
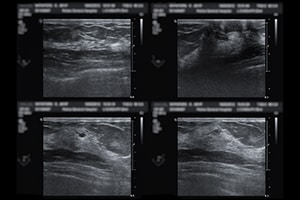
The curvature, texture, and density of the human breast presents challenges related to imaging-based screening for breast cancer. Ultrasounds are among the most accurate imaging techniques currently available, but they require highly skilled technicians, expensive machinery, or both. In a study in Science Advances, researchers designed a wearable ultrasound patch that can identify cysts as small as 0.3 cm.
The c-USB-r patch consists of a honeycomb-like mesh that attaches to a sports bra with six openings corresponding to six distinct scanning positions. A piezoelectric ultrasound array can be guided between the scanning positions using a handheld tracker. At each position, the array can be rotated 360 degrees to allow more thorough imaging of potential anomalies. According to the study authors, the patch is easier to operate than a traditional handheld ultrasound, decreasing the need for highly trained professionals.
Studies on imaging models called phantoms demonstrated a maximum field of view of 10 cm at each position and a depth of up to 8 cm. In a female volunteer with a history of cysts, the patch identified a 1 cm cyst in the left breast and a 0.3 cm cyst in the right breast, findings which were confirmed with a traditional handheld ultrasound. Scanning was later repeated on the left breast, and the 1 cm cyst remained detectable at the same position, indicating the reproducibility of results and the ability to monitor anomalies over time.
Sequencing Immune Cells Through Time and Space
Single-cell sequencing allows researchers to analyze the state and functionality of various cell types within a tumor. Fluorescent tracking allows researchers to assess how cell states change over time. Methods that can do both at the same time, however, have been lacking.
In a recent study in Cell, researchers introduced Zman-seq (an homage to zman, the Hebrew word for time), which allows for in vivo labeling of immune cells that entered the tumor microenvironment (TME) over the course of several days. The fluorescent labels—pulsed at 12, 24, and 36 hours—tag circulating immune cells before they exit the bloodstream to interact with the tumor. Exiting circulation prevents the cells from receiving subsequent tags, thereby providing an accurate estimate of when the cells entered the TME. When researchers performed single-cell sequencing on the tumors, they could tell how long each immune cell had been in contact with the tumor and use that data to map cell changes over time.
Use of Zman-seq in a mouse model of glioblastoma revealed the near-immediate induction of gene expression programs that promoted dysfunction in natural killer cells, chiefly mediated by TGFβ signaling. Researchers also traced the transformation of monocytes into immune-suppressive macrophages back to a transcription factor called TREM2; treatment with an anti-TREM2 antibody redirected this trajectory, marked by a decrease in expression of immunosuppressive and inflammatory genes. The researchers hope that detailed analysis of cell state changes within the TME can help define new tumor-promoting mechanisms and identify new therapeutic targets.
Source link
#Bench #Molecular #Jackhammers #Biobots



