Finding the Right Targets to Treat Biliary Tract Cancers
Biliary tract cancers (BTCs) have not always been easy to understand. That has been true for both patients—who might not even know about the existence of these thin tubes that transport bile from the liver and gallbladder to the small intestine until their diagnosis—as well as researchers who have struggled to develop treatment options for a cancer with a diverse mutational landscape, as described in a review published in Nature Reviews Clinical Oncology. Fortunately, that has started to change in recent years thanks to progress with molecularly targeted treatments that have led to incremental improvements in survival rates.
Incremental improvements may not sound like much, but for a cancer type with a high mortality rate, any extra time can be meaningful for patients. The majority of BTCs are cholangiocarcinomas, which are often classified as either intrahepatic—meaning the affected bile ducts are inside the liver—or extrahepatic—meaning the ducts are located outside the liver. The five-year survival rate ranges from 2% to 23%, according to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database.
In a study published in Scientific Reports, researchers in Europe wanted to look at whether targeted treatments that have entered clinical practice in recent years were leading to better overall survival. They used the European Society for Medical Oncology’s (ESMO) Scale of Clinical Actionability for molecular Targets (ESCAT) and the National Center for Tumor Diseases (NCT) Heidelberg variant classifier to determine which patients with BTC qualified for use of targeted therapy and compared their treatment to those treated with chemotherapy.
Using a retrospective chart review of patients diagnosed with BTC at five cancer centers in Austria between September 2015 and January 2022, they identified 159 patients with molecular characteristics associated with available targeted treatments. Of these, 36 patients received matched targeted treatment beyond first-line treatment, compared to 43 who were treated with cytotoxic chemotherapy. The overall survival rate for those who received targeted therapy was 22.3 months compared to 17.5 months for those who received chemotherapy, a statistically significant improvement.
Can Resistance to FGFR2 Inhibitors be Made Futile?
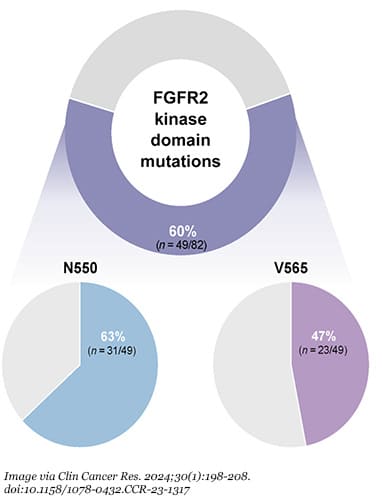
Among the targeted therapies examined in the study were fibroblast growth factor receptor 2 (FGFR2) inhibitors. Pemigatinib (Pemazyre) and futibatinib (Lytgobi) are two FGFR2 inhibitors that have been approved by the U.S. Food and Drug Administration (FDA). Genetic alterations of FGFR2, however, are only found in about 10% to 15% of intrahepatic cholangiocarcinoma (ICC) cases, and over time, patients can become resistant to treatment—often after a few months—because they develop secondary FGFR2 mutations. In a study published in Clinical Cancer Research, 60% of 82 patients with FGFR2-altered advanced ICC developed secondary FGFR2 mutations.
To overcome this issue, researchers are exploring next-generation FGFR inhibitors. During the 2024 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium in January 2024, phase II clinical trial results were announced for tinengotinib, a FGFR1-3 inhibitor that binds to FGFR in a way that blocks FGFR2 fusion and rearrangement, preventing the mutations that cause resistance to treatment. Of the patients in the trial whose tumors had developed resistance to a previous FGFR inhibitor, 37.5% demonstrated a partial response with tumor reductions ranging from 40.7% to 54.6%. A phase III trial for the drug candidate kicked off in December 2023.
Other next-generation FGFR inhibitors are in various stages of development, including RLY-4008 (phase I/II trial), erdafitinib (phase IIa), KIN-3248 (phase I/Ib), derazantinib (phase II), tasurgratinib (phase II), and HMPL-453 (phase II).
Expanded Use for PD-1 Inhibitors
Another target researchers have been studying in relation to BTC treatment is the immune checkpoint molecule PD-1. In 2017, the FDA approved pembrolizumab (Keytruda), an immunotherapy that blocks PD-1, for use in several cancer types, including all advanced solid tumors that have a high tumor mutation burden or microsatellite instability. About 5% of patients with cholangiocarcinoma have that type of tumor, according to a study published in ESMO Open.
In September 2022, the FDA approved durvalumab (Imfinzi) in combination with gemcitabine and cisplatin for adult patients with locally advanced or metastatic BTC. In the TOPAZ-1 phase III trial, this inhibitor of PD-L1 (the binding partner of PD-1) in combination with chemotherapy demonstrated a median overall survival of 12.8 months compared to 11.5 months for patients who received a placebo with chemotherapy.
The most recent FDA approval related to BTC came in October 2023 with an expanded indication for pembrolizumab to be used in combination with gemcitabine (Gemzar) and cisplatin for the treatment of locally advanced unresectable or metastatic BTC. This was based on the phase III KEYNOTE-966 trial that found patients treated with this combination therapy had a median overall survival of 12.7 months compared to 10.9 months for those who received a placebo with chemotherapy.
While both approvals expand the use of a PD-1 pathway inhibitor in BTC patients beyond those with a high tumor mutation burden, that still represents a limited number of patients. But researchers are continuing to study ways to increase the effectiveness of PD-1 inhibitors for more patients with this cancer type, for example, by pairing them with a CTLA-4 inhibitor or a tyrosine kinase inhibitor.
Monitoring More Molecular Alterations
Beyond targeting PD-1 or FGFR2, researchers are looking into other mutations found in BTC tumors as they attempt to discover more treatment options for more patients. In a study published in Clinical Cancer Research, researchers suggested the need to target BRAF mutations, which only occur in about 5% of ICC but are associated with a worse prognosis.
In KRAS-mutant mice with ICC, researchers found success through alternative splicing of the interleukin-1 receptor antagonist to block the interleukin-1 pathway, according to results published in Cancer Discovery.
Since some studies have found variants of the CD44 protein in some patients’ ICC, researchers examined the impact of using an antibody-drug conjugate therapy that targets CD44 variant 5, which is upregulated in about 30% of ICC tumors. Tumor regression was seen in xenograft models with no significant adverse toxicities from this investigational therapy, according to a study published in Cancer Research.
Another potential therapy worth keeping an eye on is BST02, which the FDA granted Fast Track Designation in January 2024 for the treatment of all types of liver cancer, including hepatocellular carcinoma and cholangiocarcinoma. If it is eventually approved, this would be the first tumor infiltrating lymphocyte (TIL) cell therapy for liver and biliary tract cancers. TIL therapies use a patient’s own immune cells from the microenvironment of the tumor to kill tumor cells. Fast Track Designation is granted to help accelerate the development and review of drugs that treat serious or life-threatening conditions.
Only about 8,000 people in the United States are diagnosed with BTCs each year, but the poor mortality rate has clearly made it a priority for researchers to find the right targets to help patients live longer.
Source link
#Finding #Targets #Treat #Biliary #Tract #Cancers



