Selpercatinib for RET-Positive Lung, Medullary Thyroid Cancers
,
by Edward Winstead
For several years, the targeted drug selpercatinib (Retevmo) has been a treatment option for some people with lung cancer or thyroid cancer whose tumors contain specific changes in a gene called RET.
But there has been limited research comparing selpercatinib with other standard treatments for tumors with RET changes. Two new clinical trials have now done just that. One trial included people with lung cancer, and the other included people with medullary thyroid cancer.
In both trials, treatment with selpercatinib prolonged the amount of time people lived before their cancers got worse, called progression-free survival, compared with other treatments.
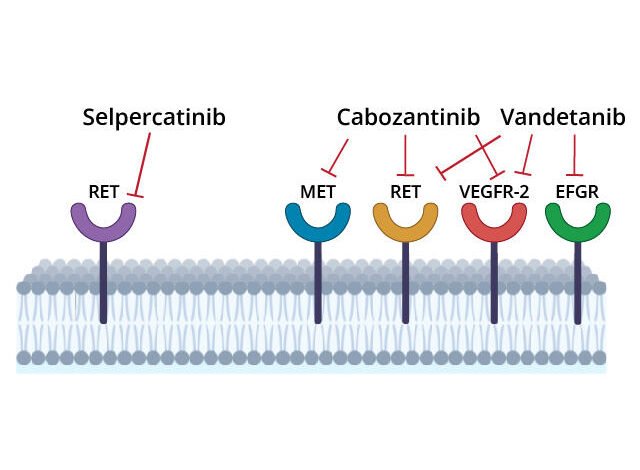
Selpercatinib is approved for treating some lung, thyroid, and other solid tumors that have RET alterations, such as mutations in the gene itself and rearrangements that fuse parts of the gene with another gene.
These mutations and fusions can result in abnormal proteins that spur cell growth and cause tumors to form. Selpercatinib, which is a pill that patients take by mouth, can block the activity of abnormal RET proteins and fusion proteins involving parts of RET.
In the lung cancer trial, LIBRETTO-431, treatment with selpercatinib more than doubled the median progression-free survival in people with advanced non-small cell lung cancer (NSCLC) containing RET fusions, compared with that in people who received chemotherapy with or without pembrolizumab (Keytruda).
And in the medullary thyroid cancer trial, LIBRETTO-531, selpercatinib was superior to two other targeted treatments based on several measures, including progression-free survival, in people with advanced medullary thyroid cancer that had RET mutations.
Results from both trials were presented on October 21 at the European Society for Medical Oncology (ESMO) Congress in Madrid and published simultaneously in the New England Journal of Medicine (NEJM).
Because of the implications of RET alterations for treatment, the findings underscore the importance of testing the tumors of people with lung cancer and medullary thyroid cancer for RET alterations at the time of diagnosis, several researchers said.
Selpercatinib superior to chemo, with or without pembrolizumab, in lung cancer
Alterations involving RET are rare, occurring in less than 1% of most cancers. About 2% of people with NSCLC have tumors with RET fusions (RET fusion–positive NSCLC).
The lung cancer trial was intended to define the most effective first-line treatment for people with advanced RET fusion–positive NSCLC. The trial was sponsored by Eli Lilly, the maker of selpercatinib.
The study’s 261 participants were randomly assigned to receive initial treatment with selpercatinib or chemotherapy, which is an initial standard treatment option. Participants in the chemotherapy group could receive pembrolizumab if their physicians recommended it. The trial was designed so at least at least 80% of participants in the chemotherapy group would get pembrolizumab.
However, it was not required because previous studies have suggested that checkpoint inhibitors such as pembrolizumab “may have limited effectiveness” for patients with RET fusion–positive NSCLC, the researchers wrote in NEJM.
At a median follow-up of about 19 months, the median progression-free survival among participants who received selpercatinib was 24.8 months, compared with 11.2 months for those in the chemotherapy group.
Tumors shrank in 84% of patients in the selpercatinib group, compared with 65% of the patients in the chemotherapy group, the researchers reported.
Based on these results, selpercatinib should be considered as a standard initial treatment for advanced RET fusion–positive NSCLC, said Herbert H. Loong, MBBS, of the Chinese University of Hong Kong, who presented the lung cancer trial’s findings at the ESMO meeting.
During a discussion of the results at the meeting, Benjamin Besse, M.D., Ph.D., of Gustave Roussy in France said that among participants who received chemotherapy, the median progression-free survival was the same—11.2 months—whether or not they received pembrolizumab.
The result suggests that “immunotherapy does not add a lot in this population,” he said.
Selpercatinib may shrink tumors in the brain
Selpercatinib was designed to cross the blood–brain barrier and to reach tumors in the brain. Previous studies have suggested that the drug may be particularly effective against tumors that have spread to the brain, as NSCLCs tend to do.
The new trial provided evidence that selpercatinib can shrink tumors in the brain in people with NSCLC. Twenty-nine participants in the trial had measurable brain metastases. During the study, these metastases shrank in 82% of those who received selpercatinib, compared with 58% of those who received chemotherapy.
The amount of time before tumors spread to the brain was longer in the selpercatinib group than in the chemotherapy group. These findings suggest that selpercatinib may prevent or delay the formation of new tumors in the brain, noted Alexander Drilon, M.D., of Memorial Sloan Kettering Cancer Center and a researcher on the study.
As in previous trials of the drug, the most common side effects of selpercatinib included hypertension, dry mouth, and diarrhea. Doctors were able to manage most of these side effects by adjusting the dose of selpercatinib, which allowed most participants to continue to receive the therapy.
Side effects of treatment led doctors to lower the dose for 51% of the participants in the selpercatinib group, versus 29% of those in the chemotherapy group. Treatment was stopped because of side effects in 10% and 2% of the two groups, respectively.
Results confirm effectiveness in medullary thyroid cancer
In the United States, medullary thyroid cancer accounts for about 2% of thyroid cancers. About 25% of people with medullary thyroid cancer have an inherited form of the disease.
RET alterations are present in nearly all hereditary medullary thyroid cancers and in up to half of sporadic forms of the disease—that is, cancers that are not the result of an inherited mutation.
Historically, advanced medullary thyroid cancer has been much more difficult to treat than other types of thyroid cancer.
The LIBRETTO-531 trial included 291 participants with RET-mutant medullary thyroid cancer that could not be removed by surgery or had spread to other parts of the body.
Trial participants were randomly assigned to receive initial treatment with selpercatinib or one of two other treatments, cabozantinib (Cabometyx) or vandetanib (Caprelsa). These drugs, called multi-kinase inhibitors, block the activity of several cancer-related proteins, including abnormal RET proteins.
Cabozantinib and vandetanib are not highly selective RET inhibitors, and the drugs have side effects that can cause patients to discontinue therapy. These limitations led Julien Hadoux, M.D., Ph.D., of Gustave Roussy and his colleagues to test whether selpercatinib, which is designed to target RET proteins only, would be more effective than cabozantinib or vandetanib. The trial was also sponsored by Eli Lilly.
At a median follow-up of approximately a year, the median progression-free survival was 16.8 months in the cabozantinib/vandetanib group. Because the cancer had not yet gotten worse in many patients receiving selpercatinib, the researchers couldn’t yet determine a median progression-free survival for the group.
Participants in the selpercatinib group were more likely to respond to the drug—that is, to have their tumors shrink—than participants in the comparison group. The treatment response rates were 69% and 39%, respectively.
The patient responses to selpercatinib were “more frequent, more profound, and more durable” than those seen in people treated with cabozantinib or vandetanib, Dr. Hadoux said at the ESMO meeting.
In the selpercatinib group, 23 patients (12%) had a complete response, meaning there was no longer any evidence of their cancers. In the control group, 4 patients (4%) had a complete response.
More precise targeting of RET leads to fewer side effects
As in the lung cancer trial, the most common side effects of selpercatinib in the thyroid cancer trial included high blood pressure, dry mouth, and diarrhea.
Treatment side effects led doctors to lower the dose for 39% of the patients in the selpercatinib group and 77% in the cabozantinib/vandetanib group. Treatment was stopped because of side effects in about 5% and 27% of the two groups, respectively.
The greater “selectivity” of selpercatinib in targeting RET likely explains why the drug has fewer side effects than cabozantinib and vandetanib, the research team wrote.
Although some oncologists are already using selpercatinib for their patients with medullary thyroid cancer, the trial results are “practice changing,” said Laura Locati, M.D., Ph.D., of the Istituto Nazionale dei Tumori in Milan, after the presentation of the results at the ESMO meeting.
“With selpercatinib we have, for the first time, a very active treatment in terms of response rate, and prolonged response, and very few side effects compared to the standard of care,” Dr. Locati added.
What’s next for targeting RET?
In the United States, genetic testing of tumors for RET alterations is standard practice for people with medullary thyroid cancer, noted Jaydira del Rivero, M.D., of NCI’s Center for Cancer Research, who studies endocrine cancers.
Based on the new results, Dr. del Rivero continued, selpercatinib should be considered the standard first-line therapy in patients with RET-positive medullary thyroid cancer.
The results, Dr. del Rivero added, demonstrate the movement “toward personalized approaches for treating thyroid cancers.”
The landscape of potential treatments for RET-altered cancers continues to evolve. Researchers are studying pralsetinib (Gavreto), another highly selective RET inhibitor. In the United States, pralsetinib is approved for the treatment of NSCLC with RET fusions and for RET-altered medullary thyroid cancers. The oral drug has a similar safety profile as selpercatinib and has also shown the ability to treat brain metastases, according to a recent study.
Meanwhile, next-generation RET inhibitors are being developed. A goal of this research, say those working on these medicines, is to develop treatments for cancers that have stopped responding to other therapies, including first-generation RET inhibitors.
Source link
#Selpercatinib #RETPositive #Lung #Medullary #Thyroid #Cancers



