FDA Approvals in Oncology: January-March 2024

With the approval of new anticancer therapeutics, more treatment options become available for patients. Some therapies are new to the market, while some may have already been approved for other indications; some molecules are first in class, directed against a previously untargeted pathway or acting through a new mechanism, while others may be improved versions of drugs that already exist.
To help our readers keep track of the cancer therapies approved by the U.S. Food and Drug Administration (FDA), understand their impact for patients, and put them in context of the current therapeutic landscape, Cancer Research Catalyst provides a quarterly review of the latest approvals from the FDA.
The FDA kicked off 2024 with a bang.
Between advances in immunotherapy, targeted therapy, and chemotherapy, the FDA issued 14 approvals for cancer indications from January through March. The slate of new indications includes four accelerated approvals, three conversions from accelerated to full approvals, and seven new full approvals.
We provide an overview of the latest approvals, including the first tumor-infiltrating lymphocyte (TIL) cell therapy to reach the market.
TIL-ling the Soil: Breaking Ground on New Immunotherapies
This quarter was marked by advances in cell-based immunotherapies and immune checkpoint inhibitors (ICIs) alike. Perhaps the most notable decision was the first ever regulatory approval for TIL therapy.
Engineering T cells to find and destroy cancer cells has been successful in several types of blood cancer but has struggled to gain traction in solid tumors. Researchers have sought to demonstrate for several years that leveraging the immune system’s own expertise—by isolating and expanding the T-cell populations that have already made it to the tumor—may provide a stronger and more durable benefit.
In TIL therapy, T cells are isolated from a tumor sample taken as a biopsy or during tumor excision. They are expanded in the laboratory and, after depleting the patient’s existing T cells to make room for new ones, infused back into the patient. The patient is then given several infusions of interleukin-2, a cytokine that promotes continued expansion and activity of T cells.
Lifileucel (Amtagvi), the first TIL therapy to reach the market, was granted accelerated approval by the FDA in February for the treatment of unresectable or metastatic melanoma. In the clinical trial that led to this approval, patients who received the recommended dose of lifileucel had an objective response rate of 31.5%, a four-year overall survival rate of 47.3%, and a median response duration that was not reached.
CAR T therapy also experienced an exciting first, with its first approval for chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Lisocabtagene maraleucel (Breyanzi), which targets the protein CD19 on the surface of B cells, was granted accelerated approval for the treatment of patients with CLL or SLL who have received at least two previous therapies, including an inhibitor of the kinase BTK and an inhibitor of the pro-survival protein BCL2.
In addition to cell-based therapies, three ICIs—drugs that block the immune system’s natural brakes to help immune cells fight cancer—were approved for new indications:
- Pembrolizumab (Keytruda), which targets the immune checkpoint protein PD-1, was approved, in combination with chemoradiotherapy, to treat patients with stage III-IVA cervical cancer. Previous approvals required cervical cancers to express the immune checkpoint protein PD-L1; exhibit high microsatellite instability, a mismatch repair deficiency, or a high tumor mutation burden; or have progressed after treatment with chemotherapy in order to receive pembrolizumab. The current approval removes these requirements.
- Nivolumab (Opdivo), which also targets PD-1, was approved, in combination with chemotherapy, for the first-line treatment of unresectable or metastatic urothelial carcinoma (bladder cancer). Nivolumab was previously approved to treat certain bladder cancers after chemotherapy or surgery and can now be used as part of a first-line regimen.
- Tislelizumab (Tevimbra), a third PD-1 inhibitor, was approved for single-agent use to treat adult patients with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) after chemotherapy given with or without a PD-1/PD-L1 inhibitor. This is the first U.S. approval for tislelizumab.
Targets Locked: Progress for Tyrosine Kinase Inhibitors
Many small-molecule inhibitors target tyrosine kinases, a family of cell signaling proteins that stimulate pathways involved in cell replication and survival. When tyrosine kinases are mutated, the pathways can become dysregulated, leading to cancer.
This quarter, tyrosine kinase inhibitors (TKIs) garnered six new approvals across several cancer types:
Non-small cell lung cancer (NSCLC)
- In February, tepotinib (Tepmetko) was approved for use in adult patients with metastatic NSCLC that harbors an exon 14-skipping mutation in the MET tyrosine kinase. Exon 14-skipping mutations cause the machinery that processes MET RNA to omit exon 14, a key piece of the protein-coding instructions. Such mutations occur in around 2% of all NSCLC tumors, resulting in dysregulated MET signaling. Tepotinib, a MET inhibitor, was previously granted an accelerated approval for this indication in 2021.
- Osimertinib (Tagrisso), an inhibitor of the epidermal growth factor receptor (EGFR), was approved, in combination with chemotherapy, for the treatment of NSCLC tumors that have certain EGFR mutations. One mutation is a deletion in exon 19, which can shorten a part of the protein that regulates the switch between EGFR’s active and inactive forms. The faulty switch favors the active form of EGFR, leading to dysregulated cell signaling and growth. The other mutation is an L858R point mutation in exon 21 which achieves a similar effect. While some EGFR inhibitors are not effective against these mutations, osimertinib is; it was previously approved as a single-agent therapy for NSCLC harboring EGFR mutations and can now be used in combination with platinum-based chemotherapy.
- Amivantamab-vmjw (Rybrevant) targets both EGFR and MET and was approved for use as a monotherapy or in combination with chemotherapy to treat NSCLC tumors with insertions in exon 20 of EGFR. These insertion mutations lengthen a different part of the same switch regulated by exon 19, similarly maintaining EGFR in its active form. Upregulation of MET signaling has been identified as a mechanism by which cancer cells develop resistance to EGFR inhibitors. Researchers hypothesized that targeting both pathways simultaneously may prevent or delay drug resistance. Amivantamab-vmjw was previously granted an accelerated approval for its use as a monotherapy in this patient population. This quarter’s decision converted the accelerated approval to a full approval and additionally approved the use of amivantamab-vmjw in combination with chemotherapy.
- In March, a treatment regimen containing zanubrutinib (Brukinsa) was granted accelerated approval for use in patients with pretreated, relapsed or refractory follicular lymphoma, a type of non-Hodgkin lymphoma that arises from B cells. Zanubrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK); BTK mutations are common in follicular lymphoma and can stimulate aberrant activation and uncontrolled growth of B cells. Zanubrutinib is the first BTK inhibitor approved for the treatment of follicular lymphoma. The newly approved regimen also contains obinutuzumab (Gazyva), an antibody that binds to CD20 on the surface of B cells, including follicular lymphoma cells, and can trigger cell death. Obinutuzumab has been previously approved as a monotherapy or as part of another targeted therapy regimen for the first-line treatment of follicular lymphoma and in combination with chemotherapy for certain patients with previously treated follicular lymphoma.
- Also in March, the FDA granted accelerated approval to ponatinib (Iclusig), in combination with chemotherapy, for the treatment of newly diagnosed acute lymphoblastic leukemia (ALL) characterized by the Philadelphia chromosome. The Philadelphia chromosome is a mutated chromosome created by a translocation between chromosomes 9 and 21, which results in a hybrid gene called BCR-ABL. BCR-ABL acts as a tyrosine kinase that isn’t subject to normal regulatory mechanisms. Ponatinib—which was previously approved to treat Philadelphia chromosome-positive ALL that did not respond to other kinase inhibitors—blocks the activity of BCR-ABL.
Bladder cancer
- Erdafitinib, an inhibitor of the fibroblast growth factor receptor (FGFR) family of proteins, was granted full approval in January to treat certain adult patients with locally advanced or metastatic bladder cancer that harbors a mutation in FGFR3. Patients must have experienced disease progression after one or more systemic therapies and, if eligible for immune checkpoint inhibitors, received prior anti-PD-1 or anti-PD-L1 treatment. In 2019, erdafitinib was granted an accelerated approval for this indication. Erdafitinib is the first FGFR inhibitor to be approved for bladder cancer.
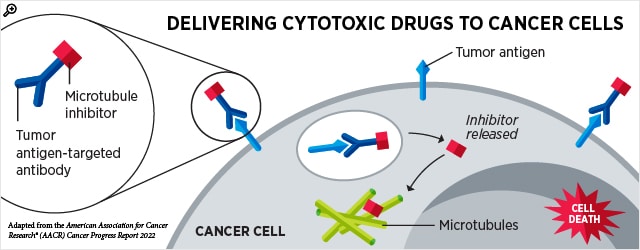
Knowing Your ADCs: Advances in Antibody-drug Conjugates
Many chemotherapy agents are designed to target cells that divide rapidly; unfortunately, this means that in addition to targeting cancer cells, they often catch rapidly growing blood and gut cells in the crossfire. Dosing becomes a tricky balance between giving enough drug to kill the cancer cells but not so much that it critically harms the patient.
Antibody-drug conjugates (ADCs) have introduced an innovative way to improve the specificity of toxic drugs by tagging them to antibodies that seek specific cell types. When the antibody binds to a protein on the surface of target cells, the ADC is taken inside the cell, where it releases the toxic payload that triggers cell death.
Two ADCs were approved for new indications this quarter:
- Inotuzumab ozogamicin (Besponsa) was approved in March for the treatment of pediatric patients with relapsed or refractory B-cell precursor ALL that expresses CD22. Inotuzumab recognizes CD22 on the surface of B cells, including dysregulated B cells responsible for some ALL cases. Ozogamicin causes catastrophic damage to DNA that leads to cell death. Inotuzumab ozogamicin was previously approved for this indication in adult patients, and the current approval allows for the treatment of patients as young as 1 year of age.
- Later in March, mirvetuximab soravtansine-gynx (Elahere) was granted full approval for patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer whose tumors express folate receptor alpha (FRα) and have become resistant to platinum chemotherapy. Mirvetuximab, the antibody portion of mirvetuximab soravtansine-gynx, targets FRα, which is often expressed in these cancers. Soravtansine interferes with normal movement of the cytoskeleton, which leads to cell death. The FDA previously granted accelerated approval to mirvetuximab soravtansine-gynx for this indication in 2022.
Making the Old New Again: Chemotherapy Updates
Chemotherapy is one of the oldest pillars of cancer care, but to this day, researchers continue to use it in new ways and to update its indications to reflect current data.
This quarter, a special formulation of irinotecan was approved—in combination with the other chemotherapeutics fluorouracil, oxaliplatin, and leucovorin—for the first-line treatment of patients with metastatic pancreatic cancer. Irinotecan liposome (Onivyde) packages the topoisomerase inhibitor irinotecan into microscopic lipid bubbles for better delivery to the pancreas.
Irinotecan liposome was approved in 2015 for the treatment of metastatic pancreatic cancer that had progressed during or relapsed following other treatments; the current approval expands the indication of irinotecan liposome to include the first-line treatment of these tumors.
In addition to introducing new ways to use chemotherapy products, the FDA also reviews the safety of chemotherapeutics and revises warnings and dosing information accordingly. In March, the FDA updated the label for the DNA synthesis inhibitor fluorouracil, which has been approved for the treatment of many cancer types dating back to 1962.
Recent research has shown that patients with the genetic disorder dihydropyrimidine dehydrogenase (DPD) deficiency are more likely to experience severe adverse events from fluorouracil. Because the enzyme DPD helps break down fluorouracil, deficiencies in DPD activity may cause fluorouracil to accumulate in the body, making its side effects worse.
The FDA has updated the labeling of fluorouracil to include a warning about its use in patients with DPD deficiency. The warning states that unusually severe or early-onset toxicities may indicate a severe DPD deficiency and that the drug should be withheld or permanently discontinued. Currently, no dose of fluorouracil has proven safe in patients with DPD deficiency.
Source link
#FDA #Approvals #Oncology #JanuaryMarch



