Babson gets one step closer to delivering on Theranos-like blood testing
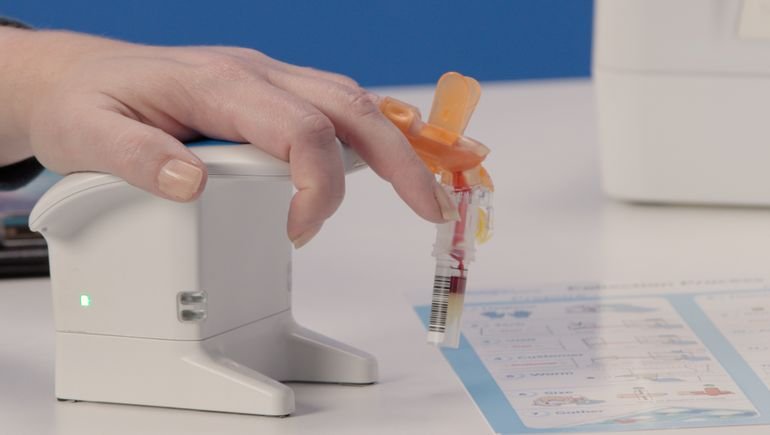
A finger-prick blood test available at the local pharmacy just got a step closer to reality. The FDA recently signed off on a key component of Babson Diagnostics’ BetterWay Blood Testing, a capillary blood collection system, developed by its strategic partner BD.
The FDA clearance positions the blood testing system to hit the market in 2024, delivering on a promise similar to the one attempted by Theranos. The now-defunct company led by CEO Elizabeth Holmes defrauded investors with false promises of a better blood test, while only delivering one of the biggest scandals in biotech history.
Building a better test
Babson, an Austin, Texas-based healthcare technology company, hopes the less invasive approach will be a friendly alternative to the traditional vial of blood taken from the arm, said Eric Olson, the company’s founder, chairman and chief operating officer. Doctor-ordered analysis will include most routine tests, complete blood count, comprehensive metabolic panel, lipid panel, vitamin D levels, thyroid stimulating hormone levels, a PSA test for men and potentially more in the future.
Babson’s approach may allow it to succeed where Theranos failed.
“We did things the right way, and we’ll take it to market once it’s ready and once it’s proven,” Olson said.
“In clinical trials, the cost of losing a patient who drops out because they can’t stand to have another needle stuck in their arm is extremely high.”

Eric Olson
Founder, chairman, chief operating officer, Babson Diagnostics
Theranos was attempting to miniaturize the lab and move it into the pharmacy to process its finger prick blood tests, Olson said.
“Babson’s strategy is to enable pharmacies to collect small capillary samples, but then create a way to stabilize and transport them to a proper laboratory where they can be run on proven equipment and overseen by medical professionals,” he said. “It’s a fundamentally different technological strategy because we’re not trying to cheat the physics and the chemistry underlying all of this by changing the way that the analysis happens.”
Unlike Theranos, which shrouded its scientific process in secrecy, Babson aims for transparency, billing itself as science-first.
“This is technology we’ve been developing since 2015, so we’ve run more than 40 different clinical trials, we’ve enrolled many thousands of subjects, and run hundreds of thousands, close to a million, blood tests as part of the validation of the system,” Olson said.
It’s also partnering with Siemens Healthineers on the initiative.
From pharmacies to pharma
If the blood testing technology makes it to market, it might remove some of the barriers that keep people from following through on doctor-ordered blood tests.
“Our core business is routine testing, where we’re concerned with making a solution that gets the 40% of people that skip their routine blood tests in to get their blood work done, which is critical to their care,” Olson said.
Babson designed its testing process for speed — it takes less than 10 minutes in and out. After collection, samples are transported by courier to one of the company’s automated micro-sample labs and patients get results within a day or two.
“Our strategy is to take this technology, the sample collection technology, the sample preparation technology, and put this into the hands of pharmacies so that pharmacies can add it to their offering,” he said. “We’re working with some big pharmacies and we’re working with some small pharmacies.”
The company is also talking with pharmaceutical companies about using the technology in clinical trials.
“In clinical trials, the cost of losing a patient who drops out because they can’t stand to have another needle stuck in their arm is extremely high,” Olson said.
The goal is to more easily capture and retain participants.
If all goes well, BetterWay will initially roll out in Texas and expand from there, potentially to organizations outside of pharmacies as well.
“We think this is a breakthrough in the way that capillary blood collection can be done because we think we can do it conveniently with the right quality to make good decisions,” Olson said. “So, there’s really a large range of applications for that kind of technology.”
Source link
#Babson #step #closer #delivering #Theranoslike #blood #testing