Antimicrobial agents in pharmaceuticals: Important considerations for formulators

Antimicrobials have a critical role to play in a long list of healthcare products. In topical preparations, antiseptic ingredients can help treat skin conditions such as cold sores, eczema, acne, and rashes. They are also essential for cleaning skin and wounds and preventing the occurrence of surgical site infections. When developing a throat lozenge, gum gel or cough syrup, antimicrobial ingredients may be chosen to help prevent infection and reduce inflammation while also preserving the formulation.
Any item with multi-dose packaging, such as eye drops and nasal sprays, are important applications for antimicrobial preservatives. Over extended use, these formulations are exposed to high microbial content, so an effective preservative has an essential role to play in maintaining the treatment’s efficacy and safety across months of use.
Broad-spectrum or targeted?
There are a range of considerations that must be made when selecting, sourcing, and formulating an antimicrobial agent. One important factor is which micro-organism/s you wish to target. Broad-spectrum antimicrobial agents are more robust and reliable, making them an easy choice for a preservative system as they provide protection from gram-positive and gram-negative bacteria, fungi, and certain viruses.
As active ingredients, a more targeted solution might be required, for instance in products aimed at a specific condition or type of infection. This can be achieved by combining different antimicrobials, adding synergistic ingredients, and adjusting the pH.
“Testing the efficacy of the antimicrobial is a crucial part of formulation design and development, where optimal concentrations and ingredient combinations will be determined. It is no small task for the formulator, as many parameters come into play, such as ingredient interactions, the formulation’s pH and physical form, and the method of delivery,”says Chantale Julien, Global Product Manager at Novo Nordisk Pharmatech.
For both active ingredients and preservatives, the antimicrobial efficacy must be tested in formulation. When the antimicrobial is used as an active, efficacy will be tested in vitro using target micro-organisms and simulating in vivo conditions. Several testing guidelines can be followed, for example the United States Pharmacopeia (USP) monograph <1072> “Disinfectants and antiseptics” and the European Pharmacopoeia (Ph. Eur.) Chapter 5.1.11 “Determination of bactericidal, fungicidal or yeasticidal activity of antiseptic medicinal products”.
For preservative systems used in multi-dose products, formulation scientists must decide which preservative and what concentration will be utilised in the drug formulation. Interactions with the drug product, as well as with the container and closure system, must be considered. The preservative must remain effective, not just ‘present’ or measurable, in the formulated product throughout its shelf life, but also at the labelled storage conditions and for the product’s ‘use period’.
The antimicrobial effectiveness test (AET), also known as the preservative effectiveness test, is a compendial test performed during drug formulation development and stability testing. Test procedures and acceptance criteria are described in the three major compendia: the USP Chapter <51> “Antimicrobial Effectiveness Testing”, the Ph. Eur. Chapter 5.1.3 “Efficacy of Antimicrobial Preservation”, and the Japanese Pharmacopeia (JP) 19 “Preservative Effectiveness Tests”. These chapters are harmonised with respect to how the test is performed, with minor differences in the selection of challenge organisms (see the table below from Moser & Meyer, 2011), test intervals, and acceptance criteria.
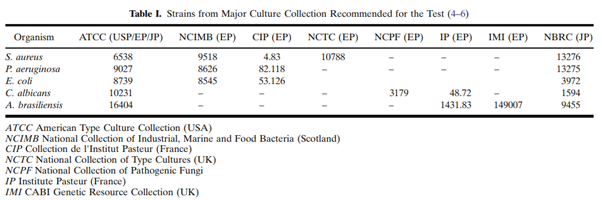
The compendial chapters divide the types of products to be tested into categories such as sterile multi-dose preparations, topical products, non-sterile oral products, etc.
Safety and sustainability concerns
As with all pharmaceutical ingredients, safety is just as important as efficacy. Researchers are becoming increasingly concerned about the contribution of antimicrobial ingredients to the rise in global bacterial resistance, which could pose a severe threat to public health in the future.
Regulators are also cracking down on the potential mutagenic, genotoxic, carcinogenic and reproductive effects of certain antimicrobials. In 2016 alone, the US Food and Drug Administration banned the use of 19 antimicrobial chemicals from household soap products.
“As an industry, we must focus on antimicrobial compounds that are effective against resistant bacterial strains and do not contribute to the burden of antibiotic resistance,” says Julien. “As much as product quality and efficacy mean to us and to our customers, they should not outweigh the importance of their safety being warranted by relevant authorities such as REACH, the US FDA, the EDQM and the WHO. At the same time, as a manufacturer and supplier, we must truly commit to act sustainably and minimise our footprint on the environment.”
While classified as non-toxic, some antiseptic ingredients can cause local irritation, especially with chronic use. Keeping irritation to a minimum is possible through the elimination of impurities during manufacturing. For example, tertiary amines are used in the manufacture of quaternary ammonium compounds (Quats). If they are present as impurities in the raw material, they can increase skin irritation. However, through careful control of the starting materials and of the manufacturing process as dictated by GMP guidelines, impurities can be kept to a minimum.
Chemicals used in pharmaceutical and personal care products such as hand disinfectants, medicated shampoos, and skincare can find their way into wastewater systems, making potential bioaccumulation a key factor in assessing the risks of ingredients.
Environmental safety is another important piece of the puzzle, particularly as pharma tightens its ESG policies. Commitment to ambitious and circular environmental policies are one way forward and must involve the whole supply chain.
Interactions and compatibility
“Ingredient compatibilities and interactions are key considerations that can impact a formulation’s efficacy and stability in both positive and negative ways,” says Julien.
In instances where a developer wishes to target their product against a specific virus or micro-organism, a clever ingredient combination can make all the difference.
To take the example of Benzalkonium Chloride (BKC), the most widely used antimicrobial from the Quats family, anti-pseudomonal effects may be enhanced through combinations with EDTA, benzyl alcohol, 2-phenylethanol and 3-phenyylpropanol. Chelators act synergistically by increasing BKC’s cell permeability, while synergy is also observed with cetrimide, 3-cresol, chlorhexidine, and organomercurials.
On the flipside, some ingredients can decrease BKC’s activity, including anionic compounds, Tween, lecithin, and others.
Is BKC the right choice?
BKC has been used as an active ingredient and preservative in pharmaceutical and personal care products for many years. It is widely available, cost-effective and presents several important features that make it a good fit for a range of applications. These include proven efficacy against a broad spectrum of bacteria, yeast, moulds, and enveloped viruses, along with stability and effectiveness at various temperature and pH levels.
Although BKC has the potential to cause local skin irritation, it is safe to use, and while many antiseptics have been banned from the market, it remains approved by authorities such as the FDA as an active ingredient in hand sanitisers.
When used as a preservative in ophthalmic and nasal formulations, BKC can also cause irritation. Following relevant guidelines such as the EDQM guideline on BKC is important when determining the preservative concentration.
The benefits of using an effective, broad-spectrum antimicrobial preservative in multi-dose formulations for non-chronic treatment outweigh the possible irritation effects. Recent unfortunate events where OTC products with severe adverse effects had to be withdrawn from the market could have been avoided by using a pure preservative of pharmaceutical GMP quality.
In addition, BKC’s environmental safety was recently investigated by P. Fuchsman et al. The researchers concluded that the chemical was not appreciably bioaccumulative and adverse ecological effects were unlikely. BKC’s effect on bacterial antimicrobial resistance (AR) was also evaluated in a thorough review by Maillard, where no AR evidence was found.
Next steps: prioritise quality
Once you have chosen an antimicrobial for your next formulation, one of the most important decisions will be where to source it from.
In the world of pharmaceuticals, it is essential to use the purest and safest ingredients on the market, and this means working with a current good manufacturing practice (cGMP) manufacturer. This way, you can minimise risks by avoiding inconsistent purity and unexpected variations while enabling easy qualification and valuable after-sales support.
As a leading supplier of ingredients to the pharmaceutical industry, Novo Nordisk Pharmatech manufactures all its products to GMP standards and complies with all relevant ICH guidelines, helping companies to minimise risk and ensure optimal processes.
Julien adds: “We are a pharmaceutical manufacturer – in this way, we operate in the same sector as our customers and we understand their challenges. We aim to keep a focus on the patients using treatments made with our ingredients, and enabling better medicines and better lives are very strong values that we live by in our everyday work.”
To learn more about working with antimicrobial ingredients in your topical formulations, you can download the whitepaper below.
Useful references and literature:
- Cheryl L. Moser and Brian K. Meyer, “Comparison of Compendial Antimicrobial Effectiveness Tests: A Review”, AAPS PharmSciTech, Vol. 12, No. 1, March 2011 (# 2011)
- Amin Omar, Patricia Nadworny, “Review: Antimicrobial efficacy validation using in vitro and in vivo testing methods”, Advanced Drug Delivery Reviews 112 (2017) 61–68
- P. Fuchsman et. Al., “Ecological Risk Analysis for Benzalkonium Chloride, Benzethonium Chloride, and Chloroxylenol in US Disinfecting and Sanitizing Products”, Environmental toxicology and chemistry, Volume41, Issue12, December 2022, 3095-3115
- J.Y. Maillard, “Impact of benzalkonium chloride, benzethonium chloride and chloroxylenol on bacterial antimicrobial resistance”, Journal of Applied Microbiology, July 2022.
- ”Benzalkonium chloride used as an excipient” (EMA/CHMP/495737/2013)
Source link
#Antimicrobial #agents #pharmaceuticals #Important #considerations #formulators
