A New Biomarker for Response to Checkpoint Inhibitors
,
by Nadia Jaber
A new study, conducted largely in mice, may help explain why a currently used molecular marker doesn’t always work to predict which patients will respond to immunotherapies called immune checkpoint inhibitors.
The study also identified another molecular marker that, if confirmed in larger studies, could one day be used as a more precise biomarker for response to these drugs.
The study focused on a feature of some cancer cells called DNA mismatch repair deficiency, which is one of several biomarkers used to determine whether certain immune checkpoint inhibitors, such as pembrolizumab (Keytruda), are likely to work for a patient.
Doctors tend to “think of mismatch repair deficiency as the best-case scenario” for predicting response to immune checkpoint inhibitors, explained the study’s lead scientist, Peter Westcott, Ph.D., of Cold Spring Harbor Laboratory in New York.
That’s because tumors that are deficient in DNA mismatch repair are chock-full of genetic mutations (called a high tumor mutational burden), and there is some evidence that the immune system has an easier time finding and killing tumors with lots of mutations.
But pembrolizumab has long-lasting effects for less than 50% of people with mismatch repair–deficient tumors. This observation and other findings have made it clear that mismatch repair deficiency and tumor mutational burden by themselves are “not very nuanced as biomarkers,” Dr. Westcott said.
The NCI-funded study found that the type and diversity of mutations in tumors appear to be more telling than the overall tumor mutational burden for predicting response to immune checkpoint inhibitors. The work appeared September 14 in Nature Genetics.
The findings came from analyses of mice with mismatch repair–deficient lung or colon tumors and data from a small number of people with mismatch repair–deficient tumors who participated in a clinical trial.
“Ideally, we’d love to have biomarkers that are 100% predictive. Unfortunately, that’s not the way biology works,” said James Gulley, M.D., Ph.D., co-director of NCI’s Center for Immuno-Oncology, who was not involved in the study.
“But anything that can give us further understanding into which patients are likely to respond to immune checkpoint inhibitors is good,” Dr. Gulley added.
Biomarkers for immune checkpoint inhibitor response
Immune checkpoint inhibitors like pembrolizumab are used to treat many types of cancer. For some people, these drugs eliminate their tumors and keep them from returning for many years. But for most people, the treatments don’t work at all or only shrink their tumors for a short time.
Immune checkpoint inhibitors are expensive, and, like all cancer drugs, they can cause serious side effects, including lung inflammation, skin rashes, and long-term thyroid problems and joint pain.
So, researchers have been searching for molecular markers that can predict whether these drugs are likely to work for a given patient. Such biomarkers can not only save patients money and help them avoid unnecessary side effects but also avoid delaying potentially more effective treatments.
Mismatch repair deficiency doesn’t always boost immunotherapy response
Mismatch repair deficiency occurs when tumor cells have a mutation in one of several genes that normally correct mistakes in the DNA code. Without that DNA spellchecker, the tumor constantly accumulates genetic mutations, leading to a high tumor mutational burden.
To investigate why some tumors with deficient mismatch repair don’t respond to immune checkpoint inhibitors, Dr. Westcott and his colleagues genetically engineered mice to spontaneously grow lung or colorectal tumors that were either deficient in mismatch repair or had functioning mismatch repair.
Tumors that were deficient in mismatch repair had many more mutations than tumors with functioning mismatch repair, the researchers confirmed.
When they treated both sets of mice with an immune checkpoint inhibitor, they found an unexpected result: mismatch repair–deficient tumors didn’t shrink any more than tumors with functioning mismatch repair.
In further experiments, the team figured out why. It came down to both the diversity and the type of mutations in the tumors, Dr. Westcott explained.
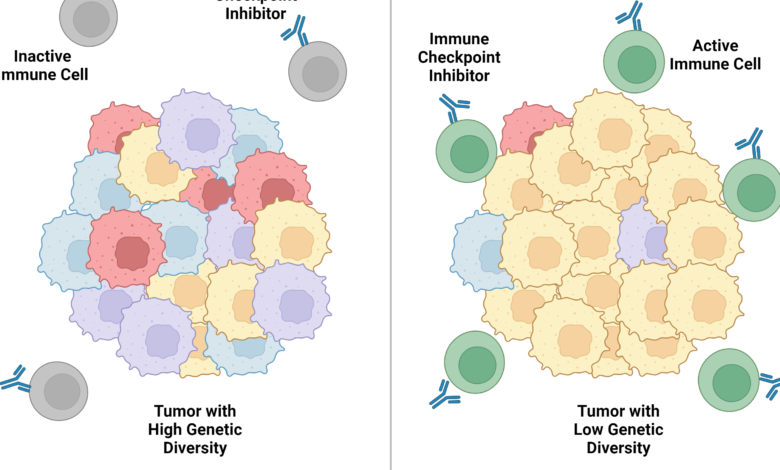
The mismatch repair–deficient tumors had a lot of genetic diversity, meaning each mutation was only in a small fraction of cancer cells. And cancer-killing immune cells couldn’t efficiently attack tumors with high genetic diversity, the researchers found.
But when they created tumors in which all of the cancer cells had the same mutations, immune checkpoint inhibitors shrank the tumors and kept them at bay for months.
The type of mutation also appeared to influence how immune system responds to tumors. Some mutations cause tumor cells to produce abnormal bits of proteins on their surface, called neoantigens. Neoantigens help the immune system spot cancer cells, whereas other types of mutations are less likely to jump-start the immune system.
Cancer-killing immune cells launched a massive attack against tumors in which all of the cancer cells had the same neoantigen, called clonal neoantigens. But that attack weakened when only a fraction of the cancer cells had the neoantigen, the researchers found.
Clonal neoantigens predict immunotherapy response
Do clonal neoantigens also matter for immunotherapy responses in humans? To answer that question, the researchers analyzed data from a small number of people with mismatch repair–deficient colorectal or stomach cancer who had been treated with pembrolizumab in a clinical trial.
People with clonal neoantigens in their tumors were more likely to have long-lasting responses to pembrolizumab, the researchers found.
Altogether, the findings suggest that the total number of clonal neoantigens—not the total number of mutations—matters most for immunotherapy response, the researchers wrote.
Other studies have reached the same conclusion, Dr. Westcott noted, though not in the context of mismatch repair deficiency.
The findings help explain why immune checkpoint inhibitors don’t shrink tumors in most people with advanced mismatch repair–deficient tumors, he added. It may well be that these treatments work best against those tumors that have both mismatch repair deficiency and clonal neoantigens, he explained.
However, the link between clonal neoantigens and the response to immune checkpoint inhibitors still needs to be tested in larger, prospective studies of people with cancer, Dr. Westcott noted.
The future of immunotherapy biomarkers
Even though mismatch repair deficiency is not a perfect biomarker, it is still useful, Dr. Gulley emphasized.
Clinical trials have found that virtually no colorectal cancer patients whose tumors have normal mismatch repair respond to pembrolizumab.
So, “you can spare people from having to get pembrolizumab if they don’t have this biomarker because they’re really not going to respond at all,” Dr. Gulley noted. “It’s saving those patients from potential side effects and added expenses.”
“Still, I don’t think anybody is satisfied with our current biomarkers,” he said.
Ultimately, whether an immune checkpoint inhibitor works for a patient rests on more than just the presence of a single genetic feature, wrote James Reading, Ph.D., of University College London Cancer Institute, and his colleagues in an editorial on the study.
“The fate of a given [patient’s] response is reliant upon a complex network” of factors, they wrote, including the immune-related components in and around tumors.
In light of this complexity, researchers are testing ways to combine multiple biomarkers, Dr. Gulley noted.
For example, some studies have shown that combining tumor mutational burden, the level of a protein called PD-L1 that blunts the immune response to cancer (and is the target of some immune checkpoint inhibitors), and the number of immune cells in the tumor is a more accurate predictor of treatment response than any of the three biomarkers alone.
Currently, Dr. Gulley said, “we don’t have something that’s black and white”—meaning, a biomarker or combination of biomarkers that can say, this patient will definitely respond.
“I don’t know if we’ll ever get there,” he said, “but I think we’ll have much better tests in the future.”
Source link
#Biomarker #Response #Checkpoint #Inhibitors



